Improved marker vaccine against classical swine fever
The present invention is a new and improved live marker vaccine for classical swine fever (CSF) in which the antigen E(rns) coding sequence is replaced with a chimeric sequence derived from two different and remotely related pestiviruses.
Classical swine fever virus (CSFV) is one of the most important animal disease pathogens in the world. The CSF vaccine most commonly used today is based on variants of the so-called C strain vaccine virus. However, this vaccine does not allow serological differentiation of infected from vaccinated animals (DIVA). A first DIVA marker vaccine from Zoetis has been approved, which is a negative marker based on the absence of an immune response against the E(rns) protein of CSFV. Still, this DIVA marker vaccine has a relatively high cross-creativity in serological tests (false positive results), most likely due to the close relationship between the pestiviruses used for vaccine development (e.g. Bovine viral diarrhea virus (BVDV) and CSFV).
Challenge
In recent years, numerous new (atypical) pestiviruses have been discovered which are genetically much less related to CSFV. The production of chimeric vaccine viruses on the basis of pestiviruses that are as remotely related as possible could be a solution here. However, chimerisation must produce efficiently replicating viruses with good protective properties which allow the production of sufficient amounts of virus.
Our Solution
Scientists at the University of Veterinary Medicine Hanover, Germany developed new marker vaccine candidates based on chimeric pestiviruses with improved DIVA properties. The inventors tested the suitability of E(rns) sequences of various remotely related pestiviruses. From the first results, a chimeric E(rns) construct comprising sequences from two distantly related pestiviruses proved to be the most promising candidate for a marker vaccine. The suitability as a protective vaccine with excellent marker properties (DIVA) was also confirmed in a first animal experiment.
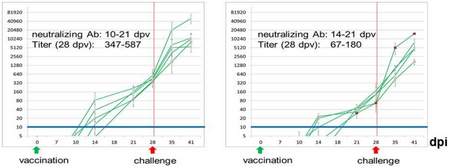
Fig.1: Determination of neutralizing antibodies induced by marker vaccine candidate 3 (RaPro). Source: A. Postel.
Fig. 2: Fig.2: Determination of DIVA properties of marker vaccine candidates. No CSFV Erns specific antibodies are detectable after vaccine application (serological negative marker)with the DIVA ELISA (lower panel), although high levels of E2 antibodies are confirmed by the conventional CSFV E2 Ab ELISA (upper panel). Source A. Postel.
Advantages
- Improved CSF marker vaccine
- No cross-creativity in serological tests
- Attenuated vaccine with good replicative and protective properties
Applications
Attenuated DIVA marker vaccine for CSF.
Development Status
First vaccination trials with stress infection were successfully carried out at the University of Veterinary Medicine Hanover.
Patent Status
Patent applications have been filed in Germany and China (DE102018110208A1, CN112020509A, Applicant: University of Veterinary Medicine Hannover, Foundation, Germany).
References
Postel et al.: Epidemiology, diagnosis and control of classical swine fever: Recent developments and future challenges. Transbound Emerg Dis. 2018, 65 Suppl 1:248-261.
Meyer et al.: Reduced specificity of Erns antibody ELISAs for samples from piglets with maternally derived antibodies induced by vaccination of sows with classical swine fever marker vaccine CP7_E2alf. Transbound Emerg Dis. 2018, 65(2):e505-e508.
Meyer et al.: The double-antigen ELISA concept for early detection of Erns -specific classical swine fever virus antibodies and application as an accompanying test for differentiation of infected from marker vaccinated animals. Transbound Emerg Dis. 2017, 64(6):2013-2022.
Contact
Dr. Stefan Uhle
Patent Manager Life Science
E-Mail: This email address is being protected from spambots. You need JavaScript enabled to view it.
Tel: +49 551 30724 154
Reference: BioC-2062-TiHo
Tags: Therapy, Life science
