CRISTAL - sample embedding for correlative microscopy
Presented here is a novel embedding procedure for correlativ microscopy, that is based upon UV-curable resins, that both fix and optically clear the specimen by optimal adjustment of the refractive index for light sheet microscopical or optical tomography (SLOT) analyses. Subsequently, the same embedded samples can be sectioned an further processed for immunohistochemical or electron microscopical analyses. In addition, the procedure allows for subsequent molecular biological analyses, such as RNA-extraction.
Challenge
|
Correlative microscopy enables the combination of various imaging techniques with each other or with subsequent molecular biological or biochemical analyses leading to a much more comprehensive analysis of one and the same specimen. It therefore represents a promising new method for biomedical research, as it for instance allows for correlation of morphological phenotypes with molecular composition of a sample or enables ultra-resolution nanoscopy of regions of interest identified in overview 3D-images. |
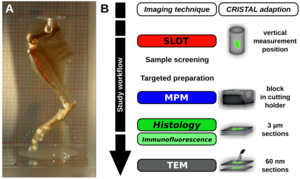
Fig. 1: CRISTAL Workflow. A) CRISTAL-embedded optically cleared leg of a mouse (Source: Dr. Ripken, LZH). B) Workflow of a correlative study. First, CRISTAL-embedded samples can be screened by using for instance SLOT. The blocks can then be sectioned according to interesting regions of interest (ROI), which can then be further investigated with for instance multiphoton microscopy (MPM) of the block surface or by histology and light or electron microscopy of the respective sections (Source: adapted from Kellner et al., 2016, Sci Rep). |
Our Solution
To solve the aformentioned problems, CRISTAL (Curing Resin-Infiltrated Sample for Transparent Analysis with Light) was developed. CRISTAL represents a novel embedding procedure for biological specimen, utilizing an UV-curable resin that contains a mercapto-ester compound (for example Norland Optical Adhesive 68 and 71). Embedding biological specimen in said medium allows for adjustment of the refractive index after polymerization in the range from n = 1,45 to n = 1,6. UV-polymerization fixes the probe, stabilizing it especially for fast movements during 3D-imaging like optical tomography (SLOT), ultra microscopy, optical projection tomography (OPT) or light sheet microscopy. Additionally, embedded specimen can be sectioned and futher be subjected to immunohistochemical analyses or electron microscopy. Furthermore, even after prolonged storage, embedded probes can be used for molecular biological techniques. As such, molecular profiling by RNA-extraction was still possible with stored samples.
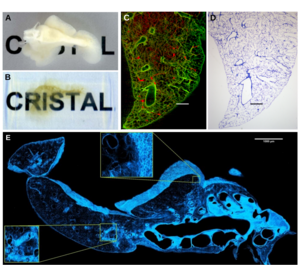
Fig. 2: Correlative microscopy of an accessory lung lobe of a rat. A) Fixed accessory lung lobe of a rat and B) after resin-based clearing (Source: adapted from Kellner et al., 2016, Sci Rep). Image correlation of an optical slice by SLOT (A) and a histological section by light microscopy (B). Scale bar: 500 µm (Source: adapted from Kellner et al., 2012, J Appl Physiol.). C) 2D MPM mosaic image of a CRISTAL-embedded accessory lung lobe of a rat on the surface of the sectioned block. Scale bar: 1 mm (Source: adapted from Kellner et al., 2016, Sci Rep).
Advantages
- sample fixation for SLOT or light sheet microscopy as well as other micro- and macroskopic imaging techniques
- optimal adjustment of the refractive index
- virtual 3D-histopathology of whole organs
- subsequent sectioning for detailed histology or electron microscopy
- protection to biodegradation, RNA-extraction possible even after longer storage periods
Applications
- correlative microscopy
- combination of 3D-imaging with histological and molecular biological techniques
- combination of 3D-imaging with electron microscopy
Development Status
- functionality proven in various tissues
- the procedure can be used immediately
Patent status
Granted german patent: DE102014108642B3
Granted US patent: US10401266B2
European patent application: EP3158311A1
Granted israeli patent: IL249598
Patent holder:
Laser Zentrum Hannover e.V.
References
Contact
Dr. Martin Andresen
Patent Manager Life Sciences
E-Mail:This email address is being protected from spambots. You need JavaScript enabled to view it.
Tel.: +49 (0) 551 30 724 150
Reference: CPA-1711-LZH
www.sciencebridge.de
Tags: Laser physics and optics