GMP-compatible Methods for producing tissue-engineered human heart muscle from stem cells
Heart tissue engineering using stem cells is a recently developed technique to construct a three dimensional cell/tissue structure from progenitor cells. Those engineered tissues can be either used for in vitro screenings (e.g. drug or toxicology screenings) or as a therapeutic tool to replace damaged or diseased tissue (regenerative medicine). Scientists at the University of Göttingen developed two new and fully defined methods for serum-free production of human engineered heart muscles (EHM) either from pre-differentiated cardiomyocytes or directly from undifferentiated stem cells. This will uniquely allow cardiological screening by measurement of muscular contraction (inotropic effects) on human tissue for the first time.
Challenge
A major challenge in tissue engineering is the need for clearly defined compounds during cell/tissue differentiation. Furthermore, laboratory-grown tissue should resemble the natural organ’s tissue as closely as possible especially with respect to its physiologic function. This is essential for qualifying as a versatile tool in preclinical drug development.
Current preclinical screening platforms rely on cardiomyocytes (single cells) and are not tissue-based. Thus, they will fail to measure muscular contraction and detection of muscular weaknesses, which is of great importance for detecting cardiological side-effects. In addition, successful industrial application will require tissue assembly under GMP-compatible protocols.
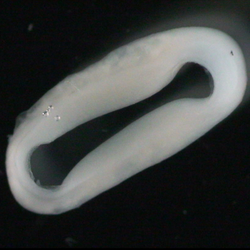
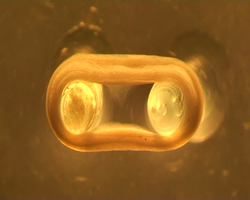 Source: M. Tiburcy
Source: M. Tiburcy
Our Solution
Scientists at University of Göttingen, Medical Department developed two new and fully defined methods for serum-free production of human engineered heart muscles (EHM) either from pre-differentiated cardiomyocytes or directly from undifferentiated stem cells. This will allow production of human EHM under strict GMP standards. Moreover, the technologies will open the doors for a new generation of cardiological screening plattforms by measurement of muscular contraction (inotropic effects) on human tissue for the first time.
Advantages
For cardiological screening (side effect or new compound screening):
- Full and quantitative measurement of muscular contraction (inotropic effects) first-time on human tissue!
- Full measurement of all other paramenters available in ordinary cardiomyocyte cellular platforms:
- Cellular and Molecular Assays for Cardiomyocyte Damage (e.g. cell viability, cell shape changes, ROS generation, apoptosis, transcriptome analysis)
- Electrophysiology Assays (chronotropic effects like beat pattern and ion channel assessment)
- Tissue engineering of small human heart muscle tissues enabling multi-well screening
- Unique measurement of muscular contraction allowing for preclinical detection of muscular weaknesses (rhabdomyolysis and myopathies),...
- ...Avoiding similar financial disasters as for the Baycol/Lipobay case with financial losses in sales (2001-forecast: 1 billion Euros) and in law cases (about 0,5 million Euros compensation since 2013)!
For regenerative stem cell approach:
- Generation of human EHM under GMP conditions using stem cell-derived material.
- Two well-defined protocols for different starting materials either from:
- pre-differentiated myocytes and non-myocytes or
- homogeneous population of undifferentiated progenitor cells.
- Human EHM have similar physiological properties to human myocardium.
Applications
In vitro:
- Toxicity Screening with special emphasis on inotropic and cardiac side effects such as arrhythmia, muscle damage, fibrosis, atrophy, hypertrophy, apoptosis, and contractile failure.
- Drug Screening for biological activity of lead compounds and identification of mechanism of action.
In vivo:
- Regenerative Medicine (replacement therapy of damaged heart myocardium).
Serum-free production of force-generating human heart muscle (EHM). Source: M. Tiburcy.
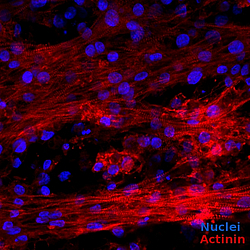
Immunostaining of EHM showing well developed muscle bundles with anisotropically aligned cardiomyocytes. Source: M. Tiburcy.
Developmental Status
The two GMP-compatible methods have been successfully established resulting in human engineered heart muscle tissue which resembles functionally native human heart muscle. Furthermore, cardiological screenings have been sucessfully performed and the screening platform is currently developed into an automated process.
Patent Status
Granted EP and US patents (Patent holder: Georg-August-University of Göttingen public law foundation).
This IP is free for license for generation of lab-grown tissues for Toxicity & Drug Screening outside Europe.
References
EP2840132B1, US10626374B2, US11492594B2
Contact
Dr. Stefan Uhle
Patent Manager Life Science
eMail: This email address is being protected from spambots. You need JavaScript enabled to view it.
Tel: +49 551 30724 154
Reference: BioC-1505-UMG & BioC-1572-UMG
Tags: Diagnostic, Therapy, Life science