A new combination therapy of pyrimidine analogues and pyrimidine biosynthesis inhibitors to combat RNA virus infections like Sars-CoV-2
This is a combined therapeutic approach for treating and/or preventing RNA viral infections using a pyrimidine biosynthesis inhibitor in combination with an antiviral pyrimidine analogue or its prodrug.
Challenge
The COVID-19 pandemic has emerged as the most serious health crisis in modern times. Its causal RNA virus, SARS-CoV-2, is a novel coronavirus presenting unique molecular, pathophysiological, and epidemiological characteristics, with a remarkable rate of contagion. Given the prevalence and severity of SARS-CoV-2 infections worldwide, the development of effective treatment protocols for COVID-19 has become a most urgent task spanning numerous fields of medical and drug research and development involving a worldwide collaborative effort. Among other potential SARS-CoV-2 therapeutic candidates, two groups of therapeutics targeting RNA viruses have been recently developed - pyrimidine analogues and pyrimidine biosynthesis inhibitors. However, the potency of these compounds at currently achievable serum concentrations has not yet been shown to effectively halt the spread and infectivity of COVID-19, especially when administered as a single dose. In fact, most of the currently available antiviral therapeutics used for treating SARS-CoV-2 infections cause severe side effects following administration and/or offer incomplete protective value (e.g. molnupiravir: only a 30 % reduction in hospitalizations and deaths).
Our Solution
The present therapeutic invention overcomes the shortcomings of known antiviral drug strategies by providing combination therapy approaches comprising one or more pyrimidine analogue and one or more pyrimidine biosynthesis inhibitor useful for the treatment of a (RNA) viral infection. This is achieved at a high level of potency at considerably lower dosages than conventional regimens, based on a surprising observation of drug synergy between these two compounds.
The present combination therapy approach is suited for viral infection caused by a number of RNA virus, e.g. by a member of the Coronaviridae virus family, an Influenza virus, HIV, an Ebolavirus, a member of the Flaviviridae comprising Hepatitis C virus, a West Nile virus, a Dengue virus, a Yellow fever virus, a Zika virus, an Enterovirus comprising Foot-and-mouth disease virus (FMDV) etc.
Advantages
- Strong drug synergy between these two compounds means:
- potential lover daily dose and hence less side effects,
- while maintaining the therapeutic effect.
- Combination of existing drugs with known pharmacokinetics, or
- Combination of novel pyrimidine analogues and pyrimidine biosynthesis inhibitors.
- Tested drugs suited for simple oral formulation (tablets).
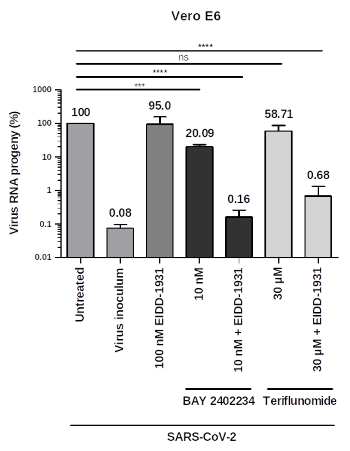
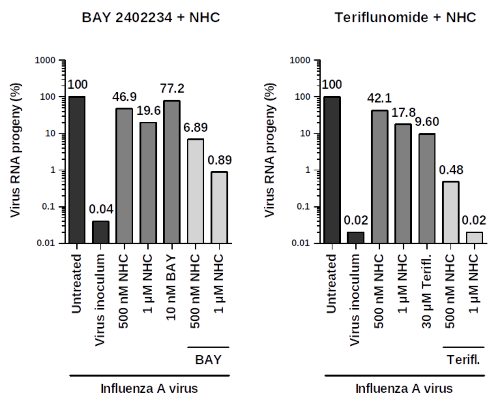 Fig.02 illustrates experimental results using combination therapeutic embodiments towards influenza A virus targeting DHODH: Combination of BAY2402234 and NHC (left) or teriflunomide and NHC (right). qRT-PCR results, virus RNA progeny in %. Virus inoculum = amount of virus used for inoculating cells. (Source: WO2022200615A1).
Fig.02 illustrates experimental results using combination therapeutic embodiments towards influenza A virus targeting DHODH: Combination of BAY2402234 and NHC (left) or teriflunomide and NHC (right). qRT-PCR results, virus RNA progeny in %. Virus inoculum = amount of virus used for inoculating cells. (Source: WO2022200615A1).
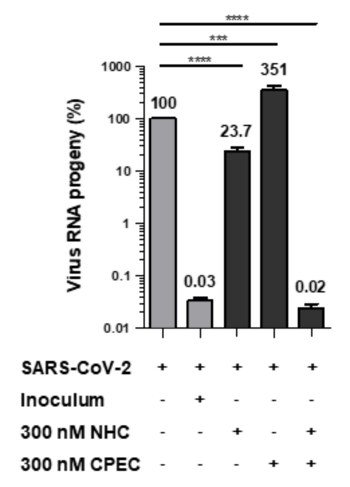 Fig.03 illustrates experimental results using a combination therapeutic embodiment towards Sars-CoV-2 virus targeting CTPS: Combination of CPEC and NHC. qRT-PCR results, virus RNA progeny in %. Inoculum = amount of virus used for inoculating cells. (Source: WO2022200615A1).
Fig.03 illustrates experimental results using a combination therapeutic embodiment towards Sars-CoV-2 virus targeting CTPS: Combination of CPEC and NHC. qRT-PCR results, virus RNA progeny in %. Inoculum = amount of virus used for inoculating cells. (Source: WO2022200615A1).
Applications
Antiviral combination therapy approach for viral infection caused by RNA viruses.
Development Status
The strong drug synergy between the compounds has been validated in vitro in at least two different cell lines with viral SARS-CoV-2 or Influenza infections. First in vivo results in suitable animal models are also available.
Patent Status
An international patent application has been filed (WO2022200615A1, applicant: Georg August University of Göttingen public law foundation).
IP is free to be (exclusively) licensed for:
- treatment of RNA virus diseases other than Sars-CoV-2 or Influenza using a combination of pyrimidine analogue with DHODH inhibitors,
- treatment of RNA virus diseases using a combination of pyrimidine analogue with CTPS inhibitors.
References
- WO2022200615A1: Pyrimidine biosynthesis inhibitor combination for use in treating viral infections.
- Stegmann et al., 2021: Pyrimidine biosynthesis inhibitors synergize with nucleoside analogs to block SARS-CoV-2 infection. bioRxiv preprint; https://doi.org/10.1101/2021.06.24.449811
- Stegmann et al., 2022: Inhibitors of dihydroorotate dehydrogenase cooperate with molnupiravir and N4-hydroxycytidine to suppress SARS-CoV-2 replication. IScience; 25(5):104293. doi: 10.1016/j.isci.2022.104293
- Schultz et al., 2022: Pyrimidine inhibitors synergize with nucleoside analogues to block SARS-CoV-2. Nature; 604(7904):134-140. doi: 10.1038/s41586-022-04482-x
Contact
Dr. Stefan Uhle
Patent Manager Life Science
E-Mail: Diese E-Mail-Adresse ist vor Spambots geschützt! Zur Anzeige muss JavaScript eingeschaltet sein!
Tel.: +49 551 30724 154
Referenz: BioC-2319+2359-UMG
Tags: Therapy, Life science
