1-vector otoferlin DFNB9 gene therapy
Mutations affecting the OTOF gene lead to severe non-syndromic form of prelingual or temperature-sensitive hearing impairment DFNB9. Due to the large OTOF size a one-vector delivery has remained challenging.
The technology offered: Gene-therapy of the otoferlin gene (OTOF) with overloaded AAV virus mediated one-vector delivery of full-lenght otoferlin or active fragment thereof into the cochlea. In vivo proof-of-concept successfully achieved. We filed international IP rights and are looking for a licensing partner to develop and market a product.
Challenge
Deafness challenge: Molecular diagnostics have identified novel deafness genes, but no causal treatments are clinically available. The sole therapeutic option is the earliest possible fitting with hearing aids or cochlear implants. Gene therapy is emerging as a promising tool to correct faulty gene expression.
Otoferlin vs. deafness: Mutations affecting the OTOF gene lead to severe non-syndromic form of prelingual or temperature-sensitive hearing impairment DFNB9. OTOF-related deafness accounts for up to 8% of autosomal recessive non-syndromic hearing loss in some Western populations. OTOF-related deafness resides within the top five of genetic hearing disorders that still require therapeutic intervention.
Otoferlin challenge: It is abundantly expressed in sensory inner hair cells (IHCs) of the cochlea. It serves as a key role in the final steps of synaptic vesicle fusion at cochlear hair cell synapses with afferent spiral ganglion neurons. To date, several OTOF mutants are known. Otoferlin deletion mice mutants are completely deaf. Due to the large OTOF coding sequence (5991 kb) and the restricted packaging size of standard adeno-associated viral vectors (AAV, <4.4 kb) that are commonly employed for gene therapeutic applications based on their favorable safety profile, the delivery of the large full-length OTOF gene has remained challenging.
Our Solution
We offer: an overloaded AAV virus one-vector delivery of full lenght otoferlin (or active fragments thereof) into the cochlea for treating OTOF related deafness. We achieved a successful in vivo proof of principle showing hearing rescue. Thus, Otoferlin genetic rescue is indeed feasible and exerts clinical potential.
Recently new capsid designs have been developed to carry larger genes. The innovative construct is using an AAV/PHP.B vector carrying the full-length otoferlin-cDNA under the control of the CAG-promoter.
Highlights: Injection into the cochlea of early postnatal mice (p5-7). The transduction of otoferlin knock-out mice (Otof-KO) with the innovative construct achieved:
- Clinically relevant transduction rates of IHCs.
- Produced adult animals with measurable auditory brainstem response thresholds.
- No side-effects did appear.
- The innovative construct might form a solid basis for the further development of gene replacement therapy for DFNB9 patients.
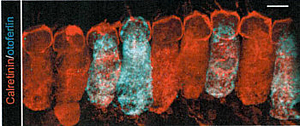
The early postnatal (p5-7) transduction of otoferlin knock-out mice (Otof-KO) with the innovative construct achieved clinically relevant transduction rates of Inner Hair Cells (IHCs). The figure shows AAV PHP.B-mediated exogenous expression of otoferlin in Otof-KO mouse inner hair cells. Calretining was used as a counterstain to specifically label IHCs. Scale bar 5 microm.(source: Moser, Rankovic, Vogl, Weber)
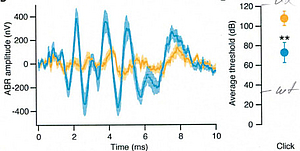
The early postnatal (p5-7) transduction of otoferlin knock-out mice (Otof-KO) with the innovative construct produced adult animals with measurable Auditory Brainstem Recording (ABR) thresholds. In vivo auditory brainstem response recordings of adult, postnatally-injected Otof-KO mice reveal successful rescue of ABR amplitudes and average click thresholds. (blue: AAV-injected, therapy; yellow: non-injected.) (source: Moser, Rankovic, Vogl, Weber)
Advantages
- One-vector system for Otoferlin based gene therapy.
- Simpler to be produced by companies.
- Easy to be used for patients and easyly administered by doctors.
- Easy to be received by patients.
Applications
For restoration of hearing in deafness DFNB9 patients (with any mutation/s in the otoferlin gene).
Developmental Status
In vivo proof-of-concept successfully achieved.
Patent Status
International IP rights applied for in the name of the Georg-August-Universität Göttingen Stiftung Öffentlichen Rechts, Universitätsmedizin.
Contact
Dr. Martin Andresen
Patent Manager LifeSciences
E-Mail: Diese E-Mail-Adresse ist vor Spambots geschützt! Zur Anzeige muss JavaScript eingeschaltet sein!
Tel.: +49-551-30724 150
Reference: BioT-2085-UMG
Tags: Therapie
