Pharmacologically controlled vector for CNS gene therapies
Current gene therapy is irreversible and does not allow controlled transgene expression in case of side effects. The invention is a pharmacologically controlled one-vector expression system of a therapeutic factor (i.e. GDNF) with zero background expression, based on mifepristone (Mfp)-Gene Switch system arranging two cassettes.
Challenge
Gene therapy in its current configuration is irreversible and does not allow controlled transgene expression in case of side effects. Only few regulated vector systems are available. Popular Tet-operon based systems show undesired immunigenic problems. Two vector systems, originally developed for safety control, are unlikely to be approved for human gene therapy. Our innovation enables a pharmacologically controlled one-vector expression system of neurological factors.
Our Solution
The technology is a pharmacologically controlled one-vector expression system of a therapeutic factor (i.e. GDNF) with zero background expression, based on mifepristone (Mfp)-Gene Switch system arranging two cassettes in tail-to-head configuration (EP3235516): (1) a first expression cassette directing the expression of a regulator protein under the control of a first promoter, wherein the regulator protein is activated in the presence of an activator molecule, and (2) a second expression cassette directing the expression of a molecule of interest (neurological factor), comprising a promoter region, and the expression of the molecule of interest is induced by binding of the activated regulator protein to the promoter region.
It has been successfully tested in vivo for Parkinson's disease (PD) and Huntington Disease. Preliminary in vivo R&D in Molec Therap 2012, 20, 534; Molec Therap 2013, 2, e106; Neurobiol Dis 2014, 65, 35.
It can be build with:
- Several constitutive promoters possible (which may be cell-type specific or ubiquitosly active), i.e. glial fibrillary acidic protein (GFAP), human synapsin 1 gene (hSYN1)..
- Several regulator proteins possible, i.e. GAL4 DNA.
- Several activator molecules possible, i.e. mifepristone (Mfp).
- Optionally further components.
- Includes a therapeutically active oligo- or polynucleotide, i.e. neurotrophic factor, such as GDNF.
- Several adeno-associated-virus, i.e. AAV5.
Scientific Background
In vivo proof of an efficient gene therapy for Parkinson's Disease (PD) using astrocytes as hosts for localized neurotrophic factor delivery has been achieved. The expression is achieved by a viral vector with enhanced tissue spread. The studies showed that astrocyte expressed glial cell line-derived neurotrophic factor (GDNF) provides in vivo restoration of motor performance in a clinically relevant lesion paradigm (Molec Therap 2012, 20, 534-543).
An in vivo adeno-associated virus (AAV)-mediated, Mifepristone-regulated Gene Switch system for the transgene expression of glial cell line-derived neurotrohic factor (GDNF) in the rat brain/CNS has been achieved. However it showed considerable background expression in the non-induced state (Molec. Therap. 2013, 2, e106).
Studies demonstrates pre-clinically in a rat PD model that short-term induced expression of glial cell-line derived neurotrophic factor (GDNF) is sufficient to provide: (i) substantial protection of nigral dopaminergic neurons from degeneration, and (ii) restoration of dopamine supply and motor behaviour in the partial stratial 6-OHDA model of PD. Monthly application of the inducing drug Mfp was sufficient to maintain neuroprotective and neurorestorative GDNF levels. The system can be induced consecutively several times. Moreover, the pharmacologically controlled system shows in vivo that discontinuous GDNF gene therapy restores motor function in a rat model of Parkinson's disease (Neurobiol. Dis. 2014, 65, 35-42).
Mechanism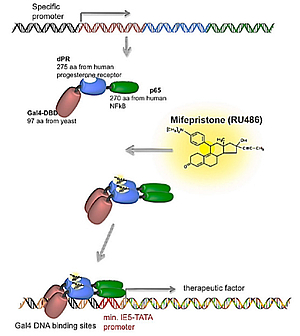
The system is activated by binding of the activator molecule being the synthetic small molecule steroid mifepristone (Mfp) to ta truncated human progesterone receptor, which is fused to a human p65 transactivated domain and a yeast Gal DNA-binding domain. Upon binding of Mfp to the Gene Switch (GS) fusion protein, it dimerizes and binds to a polynucleotide sequence consisting of several GAL4 DNA binding sites. Consequently, the p65 moiety of the GS fusion protein recruits the basic cellular transcription machinery, allowing expression of the therapeutic factor from a minimal promoter. Withdrawal of Mfp disables binding of the GS fusion protein to its DNA target sequence, and thus cease transgene expression. The system is suitable for neurotrophic factor-based gene therapy.
Experimental Results
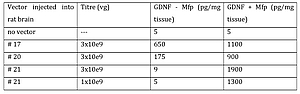
Several different configurations of a one-vector GS-GDNF layout were designed, which were packaged into AAV-5 viral capsides and tested in the rat brain for GDNF production in absence or presence of Mfp. Table 1 shows GDNF levels obtained from rat brains before and after induction with Mfp. Lead candidate is #21.
Moreover, at four weeks after Mfp induction, GDNF levels in rats injected with AAV-5 #21 at 1.10e9 vg had returned to background levels again. To our knowledge, this is the first description of any regulated gene transfer system in AAV vectors with zero background expression in the non-induced state, and the first functional one-vector genome layout of the gene switch system in a AAV vectors.
Advantages
- One-vector systems.
- Human components.
- Activator is a clinically approved small drug (Mfp).
- Tested in the rat CNS.
- Control through discontinuous administration of activator molecule (Mfp) to control induction of expression of the therapeutically active molecule (i.e. GDNF).
- Human equivalent dosage of Mfp is lower than psychiatric treatment doses.
- It prevented in vivo neurodegeneration in Parkinson's disease (PD).
Applications
- Treating, ameliorating or preventing a disease.
- I.e. Parkinson's Disease, Huntington's Disease, amyloid related disorders, spinal cord lesion.
Developmental Status
Successfull in vivo proof of concept for Parkinson's Disease and Huntington Disease achieved.
Patent Status
IP rights (EP3235516A1, US2017304464A1) have been filed in the name of the University Medical Center of Göttingen and a licensing partner is sought.
References
Preliminary in vivo R&D in Molec Therap 2012, 20, 534; Molec Therap 2013, 2, e106; Neurobiol Dis 2014, 65, 35.
Contact
Dr. Martin Andresen
Patent Manager Life Sciences
E-Mail: mandresen@sciencebridge.de
Tel.: +49-551-30724150
Reference: BioT-1922-UMG
Tags: Therapie
