Raman-Refraktometer Analyseautomat
Die folgende Erfindung stellt eine Möglichkeit, Infusionen bei der Verabreichung durch eine Spritzenpumpe zu identifizieren. Die Methode sieht eine Kopplung aus Raman-Spektroskopie und Refraktometrie vor. Beide verwendeten Analysemethoden entstammen der instrumentellen Analytik. Weiterhin werden für die Auswertung Algorithmen verwendet, die dem indirekten Hardmodelling und der Statistik zugeordnet werden können.
Problemstellung
Das Problem der fehlerhaften Verabreichung von Medikamenten und Infusionslösungen im Krankenhäusern ist bekannt. Bei einer eindeutigen Identifikation, wofür sowohl die Raman-Spektroskopie als auch die Refraktometrie nötig sind, lassen sich Fehlmedikationen von der hier vorgestellten Erfindung erkennen und ansschließend vom medizinischen Fachpersonal verhindern. Bei dem Abgleich des Ergebnisses der Analyse mit der Krankenhaus-EDV ist ein weiterer Service (wie z.B. die Medikamentenunverträglichkeit) vorhanden, die eine Alarmierung triggern kann. Bisher beruhen alle Versuche, Fehlmedikationen zu reduzieren, auf nicht-technischen Lösungsansätzen. So z.B. die Anwendung von Barcode-Systemen.
Unsere Lösung
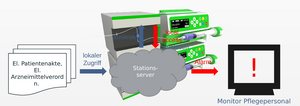
(Abbildung 1.Studie eines automatisierten Analysators zur Identifizierung der Infusionslösung in der Spritze)
Die Medikamente, die unter anderem auch in Lösungen vorliegen, werden innerhalb einer Durchflussmesszelle Raman-spektroskopisch und refraktometrisch analysiert. Die Ergebnisse der beiden Methoden werden miteinander kombiniert und mit mit der Spektren- und Brechungsindex-Datenbank abgeglichen. Dies stellt ein erstes Ergebnis dar, welches durch den Analyseautomaten ausgegeben wird. Die Ausgabe des erweiterten Endergebnisses erfolgt nach Abgleich des ersten Ergebnisses mit der Krankenhaus-EDV. Eine solche Messstrategie wird dadurch ermöglicht, dass viele Medika-mente allein mittels der Raman-Spektroskopie bereits detektierbar sind. Allerdings können wässrige Lösung von NaCl und KCl nicht durch die Raman-Spektroskopie unterschieden werden. Hier kommt die Refraktometrie zum Einsatz, die diesen Nachteil ausgleicht. Damit sind die in der Medizin eingesetzten wässrigen Lösungen von NaCl und KCl eindeutig voneinander unterscheidbar. Im nächsten Schritt erkennen Algorithmen mittels indirektem Hardmodelling und statisti- schen Methoden nicht nur die Zusammensetzung an Medikamenten in der Lösung, sondern auch die Mengen. Das Ergebnis der Raman-Messung ermöglicht eine Qualifizierung und Quantifizierung der Raman-aktiven Substanzen. Die Refraktometrie dient in diesem Fall der Verifizierung des Raman-Ergebnisses. Das Ergebnis der Quantifizierung wird gleichzeitig optimiert. Für Raman-inaktive Substanzen ist dies nicht direkt möglich. Hier obliegt die Qualifizierung und Quantifizierung der Refraktometrie.
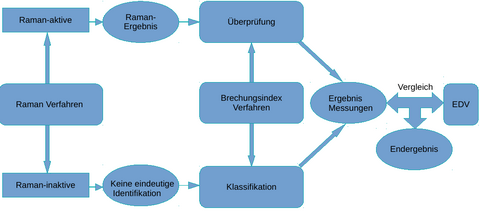
(Abbildung 2.: Schematische Darstellung der Datenfusion und des Vergleichs mit einer internen Datenbank und optionaler Vergleich mit der Krankenhaus-IT über das anzuwendende Medikament.)
Vorteile
- direkte Analyse der Medikation
- Erkennung von Fehlmedikationen
- Reduktion menschlicher Fehler
Anwendungsbereiche
- im Medizinbereich, Überwachung von Infusionen
- Einsatz in der Petrochemie
- Einsatz in der Lebensmittelindustrie
- generelle Möglichkeit von Qualitätskontrollen in Flüssigkeiten
Entwicklungsstand
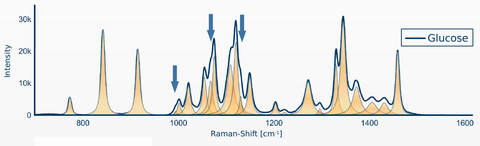
Die (Abbildung 3) zeigt ein Beispiel für die Raman-Signalerkennung von Glukose. Darüber hinaus ist die automatisierte Detektion der folgenden Substanzen wie: Insulin 1i.E. Noradrenalin, Heparin, NaCl, KCl, NaBic bereits erfolgreich realisiert. Es existiert auch ein finaler Prototyp. Der nächste Schritt ist die Entwicklung eines kommerziellen Gerätes.
Patentsituation
Deutsche Patentanmeldung: DE102017108120A1 (offengelegt)
Europäische Patentanmeldung: EP3610245A1 (offengelegt)
US Patentanmeldung: US10871399B2 (erteilt)
Patentinhaber/-anmelder:
Institut für Nanophotonik Göttingen e.V.
Kontakt
Dr. Maria Kamper
Patentmanger (Physik, Technik und Software)
E-Mail: Diese E-Mail-Adresse ist vor Spambots geschützt! Zur Anzeige muss JavaScript eingeschaltet sein!
Tel.: +49 (0) 551 30 724 159
Referenz: CPA-1980-LLG
www.sciencebridge.de
Tags: Mess- und Analysetechnik, Physik und Technik & Software
